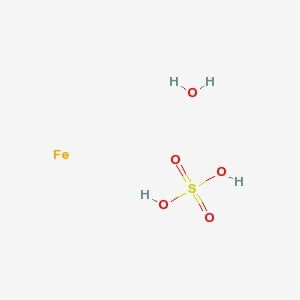
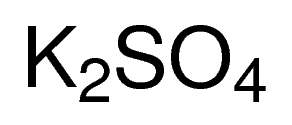Ferrous Sulfate Heptahydrate
CAS NO: 7782-63-0
MOLECULAR FORMULA :- FeSO4.7H2O
MOLECULAR WEIGHT :- 278.02 g/mol
SPECIFICATION IP BP USP Description Bluish green crystals or a light green, crystalline powder, odourless.Efforescent in air. On exposure To moist air, the crystals rapidly oxidize and become brown. Bluish green crystals or a light green, crystalline powder,efforescent in air. Bluish green crystals or a light green, crystalline powder, odourless.Efforescent in air. Solubility – Freely soluble in water, very soluble in boiling water, practically insoluble in ethanol (96 %) Ferrous sulfate heptahydrate is oxidized in moist air, becoming brown. – pH 3.0-4.0 ( 5 % w/v Solution) 3.0-4.0 – Appearance of Solution The solution is not more opalescent than opalescent Standard. – – Identification (A) Reaction
(B) Reaction
(C) Assay
A. It gives reaction of sulfates B. It gives reaction of Ferrous Salt
A. It gives reaction of sulfates B. It gives reaction of Irons
C. It complies with limit of assay.
A. It gives reaction of sulfates B. It gives reaction of Ferrous Salt
Chloride NMT 250 ppm NMT 200 ppm – Basic sulphate Producing solution that is not more than turbid. – – Arsenic NMT 2 ppm – NMT 3 ppm Lead NMT 50 ppm – NMT 10 ppm Mercury – – It meets the requirements of the test for mercury Chromium – NMT 50 ppm – Copper NMT 50 ppm NMT 50 ppm – Ferric ions NMT 0.5 % NMT 0.3 % – Manganese NMT 0.1 % NMT 0.1 % – Nickel – NMT 50 ppm – Zinc NMT 500 ppm NMT 50 ppm – Organic volatile impurities – – Meets the requirements. Residual Solvents – – Meets the requirements. Assay 98.0 % – 105.0 % 98.0 % – 105.0 % 99.5% -104.5 % Magnesium Chloride Hexahydrate
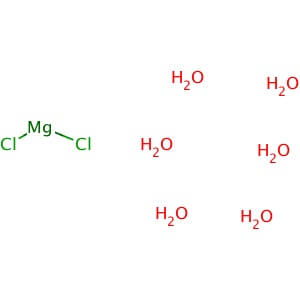
CAS NO: 7791-18-6
MOLECULAR FORMULA: MgCl2•6H2O
MOLECULAR WEIGHT: 203.30
Application: Magnesium Chloride, Hexahydrate is a source of magnesium ion and a co-foactor for many enzymes Purity: >99% Molecular Weight: 203.3 Molecular Formula: MgCl2•6H2O Description: Magnesium Chloride, Hexahydrate is widely used as a source of magnesium ion in chemistry and molecular biology applications. In biological systems, magnesium is a co-factor for many enzymes including deoxyribonuclease (DNase) and various restriction enzymes. Also plays a role in cell membrane integrity, muscle cell physiology, cardiovascular and muscular activity, and nucleic acid structure. Magnesium chloride solution is a favorable choice as an elution buffer for antibody affinity column purifications; it is much milder on most antigens than acid elution, allowing reuse of the antigen column. Also an essential cofactor for the DNA polymerase in polymerase chain reaction (PCR) amplification. Physical State : Solid Solubility : Soluble in water (20 mg/ml), and alcohol. Storage : Store at room temperature Melting Point : 116-118° C (dec.) Density : 1.57 g/cm3 at 20° C Magnesium Sulfate Heptahydrate
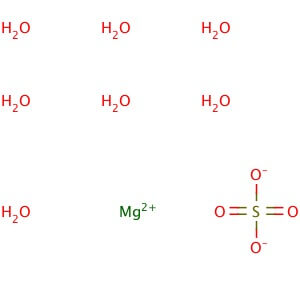
CAS NO: 10034-99-8
MOLECULAR FORMULA :- MgSO4.7H20
MOLECULAR WEIGHT :- 246.50 g/mol
SPECIFICATION IP BP USP Description Colorless crystals or white, Crystalline powder. White or almost white, crystalline powder or brilliant , colorless crystals. Colorless crystals or white, Crystalline powder Solubility – Freely Soluble in water , very soluble in boiling water and practically insoluble in ethanol (96 % )
Identification (A) Reaction
(B) Reaction
A : Reactions of Sulphates B : Reactions of Magnesium salts
A: It gives reaction of Sulphates. B :It gives reaction of magnesium
A: It gives reaction of Sulphates. B : It gives reaction of magnesium
pH of a 5% solution at 250C – – 5.0 – 9.2 Appearance of solution Dissolve 5 g in sufficient carbon dioxide free water to produced 50 ml (Solution A) is clear and colorless. Dissolve 5 g in sufficient carbon dioxide free water to produced 50 ml (Solution S) is clear and colorless. – Acidity or alkalinity NMT 0.2 ml of either 0.01 M hydrochloric acid or 0.01 M sodium hydroxide is required to change the color of solution. NMT.0.2 ml of 0.01 M hydrochloric or 0.01 M sodium hydroxide is required to change the color of indicator. – Arsenic NMT 2 ppm.
NMT 2 ppm – Heavy metals NMT 10 ppm.
– NMT 10 ppm Selenium – NMT 30 ppm Iron NMT 200 ppm. NMT 20 ppm NMT 20 ppm Chloride NMT. 300 ppm NMT. 300 ppm
NMT. 140 ppm Organic Volatile impurities – Meets the requirement Residual Solvents – Meets the requirement Loss on drying 48.0 to 52.0 %
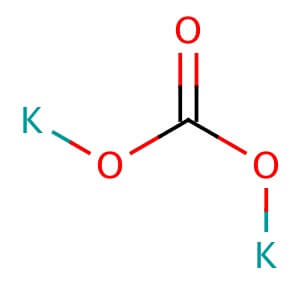
48.0 % – 52.0 % NMT 2.0 % Loss on Ignition – – 40.0%-52.0% Assay (on dried basis) 99.0 % to 100.5 % 99.0 % – 100.5 % 99.0%- 100.5% Potassium Acetate
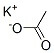
CAS NO: 127-08-2
MOLECULAR FORMULA :- C2H3KO2
MOLECULAR WEIGHT :- 98.14 g/mol
SPECIFICATION BP USP Description White or almost white, crystalline powder or colorless cryastals,deliquescent. White or almost white, crystalline powder or colorless cryastals,deliquescent. Solubility Very soluble in water, freely soluble in ethanol (96 %) – Identification A. Reaction
B. Reaction
A. Reaction of acetates.
B. Reaction of potassium
A. Reaction of acetates.
B. Reaction of potassium
Appearance of solution Clear & Colorless – pH 7.5-9.0 7.5-8.5 Heavy metals – NMT 20 ppm Limit of Sodium – NMT 0.03 % Reducing substances The solution remains pink. – Chloride NMT 200 ppm – Sulfates NMT 200 ppm – Aluminum NMT 1 ppm – Iron NMT 20 ppm – sodium NMT 0.5 % – Loss on drying NMT 3.0 % NMT 1.0 % Residual Solvents – Meets the requirements Assay 99.0 %-101.0 % 99.0%-100.5 % Potassium Carbonate Anhydrous
CAS NO: 584-08-7
MOLECULAR FORMULA :- K2CO3
MOLECULAR WEIGHT :- 138.21 g/mol
SPECIFICATION BP USP Description White or almost white granular Powder hygroscopic. White or almost white granular Powder hygroscopic. Solubility Freely Soluble in water, practically insoluble in Ethanol (96%) – Identification (A) Reaction
(B) Reaction
(C) Reaction
A : The solution is strongly alkaline B: Test A gives the reaction of Carbonates &Bicarbonates.
C: Test A gives reaction of Potassium.
A: Test A gives the reaction of Carbonates. B: Test A gives reaction of Potassium.
Insoluble substances – The solution is complete, clear & Colorless. Heavy metals – NMT 5 ppm Calcium NMT 100 ppm – Iron NMT 10 ppm – Chlorides NMT 100 ppm – Loss on Drying NMT 5.0 % NMT 0.5 % Sulphates NMT 100 ppm – Organic Volatile Impurities – Meets the requirement Residual Solvents – Meets the requirement Assay 99.0 % – 101 % (on dry basis) 99.5 % – 100.5 % Potassium Chloride
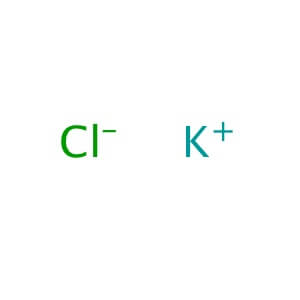
CAS NO: 7447-40-7
MOLECULAR FORMULA: KCl
MOLECULAR WEIGHT: 74.55
Alternate Names: Potassium Chloride is also known as KCl. Application: Potassium Chloride is a common laboratory reagent and calibration standard for measuring electrical conducivity. Purity: >99% Description: Potassium Chloride is commonly used as a laboratory reagent as a standard. Potassium Chloride may be used as a calibration standard ionic solution for measuring electrical conductivity because Potassium Chloride solutions, when prepared carefully, produce repeatable measurable properties. Potassium Chloride is also a good source of ionic chloride, which should precipitate insoluble chloride salts upon addition to a solution of an appropriate metal ion. Oral Potassium Chloride is commonly used to replenish vital potassium in the body, and has also been shown to lower blood pressure. Potassium Chloride is commonly used in fertilizer. Physical State : Solid Solubility : Soluble in water (340 mg/ml at 20° C), alcohol (slightly), and ethanol (1 g/250 mL). Insoluble in ether, and acetone. Storage : Store at room temperature Melting Point : 770° C (lit.) Boiling Point : 1500° C (lit.) Density : 1.98 g/cm3 at 25° C (lit.) Refractive Index : n20D 1.49 Ki Data : CA IV: Ki= 90 µM (human); CA I: Ki= 6 mM (human); CA IX: Ki= 33 mM (human); CA V: Ki= 156 mM (human); CA II: Ki>200 mM (human) Potassium Citrate
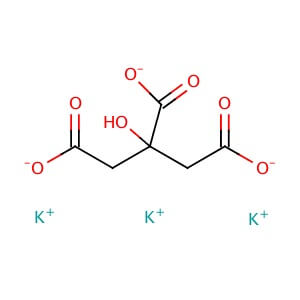
CAS NO: 866-84-2
MOLECULAR FORMULA: C6H5K3O7
MOLECULAR WEIGHT: 306.39
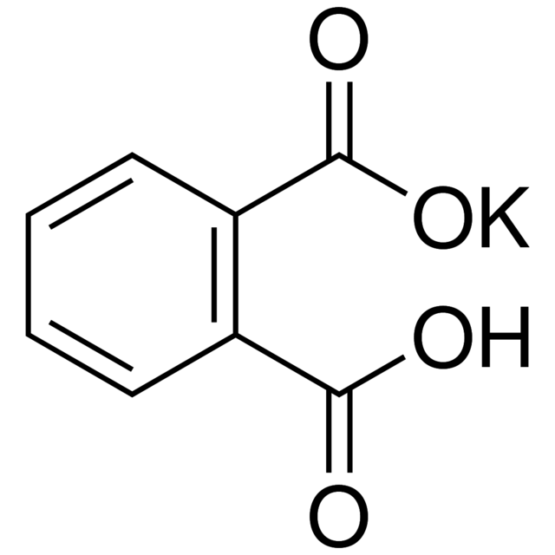
SPECIALITY FINE CHEMICALS (AR / LR / ACR /GR / IP / BP / USP / FSSAI )Potassium Hydrogen Phthalate
CAS NO: 877-24-7
MOLECULAR FORMULA : C8H5KO4
MOLECULAR WEIGHT : 204.22
SPECIALITY FINE CHEMICALS (AR / LR / ACR /GR / IP / BP / USP / FSSAI )Potassium Nitrate
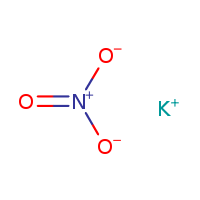
CAS NO: 7757-79-1
MOLECULAR FORMULA :- KNO3
MOLECULAR WEIGHT :- 101.1g/mol
SPECIFICATION BP USP Description White or almost white, crystalline powder or colorless crystals. White or almost white, crystalline powder or colorless crystals. Solubility Freely soluble in water, very soluble in boiling water, practically insoluble in ethanol (96 %) – Identification A. Reaction
B. Reaction
A. It gives reaction of nitrates. B. It gives reaction of Potassium
A. It gives reaction of nitrates. B. It gives reaction of Potassium
Acidity or Alkalinity NMT 0.5 ml of 0.01 M HCl or 0.01 M NaOH is required to change the color of the indicator. – Reducible Substances The solution does not become blue within 2 min. – Chlorides NMT 20 ppm NMT 300 ppm Sulfates NMT 150 ppm NMT 1000 ppm Limit of Nitrite – NMT 5 ppm Heavy metals – NMT 20 ppm Arsenic – NMT 3 ppm Lead – NMT 10 ppm Ammonium NMT 100 ppm – Calcium NMT 100 ppm – Iron NMT 20 ppm NMT 10 ppm Residual Solvents – Meets the requirements. Sodium NMT 1000 ppm NMT 1000 ppm Loss on Drying NMT 0.5 % – Assay 99.0 %-101.1 % (on dried basis) 99.0 %-100.5 % Potassium Phosphate Monobasic
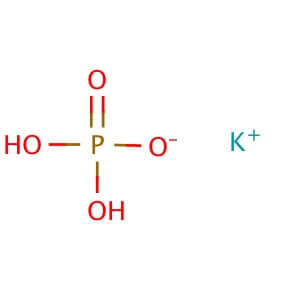
CAS NO: 7778-77-0
MOLECULAR FORMULA :- KH2PO4
MOLECULAR WEIGHT :- 136.10 g/mol
SPECIFICATION IP BP USP Description White or almost white powder , crystalline powder , colorless powder or crystalline masses, efflorescent. White or almost white powder , crystalline powder , colorless powder or crystalline masses, efflorescent. Appearance of solution 4% w/v Solution is clear and colorless – Solubility Soluble in water , very soluble in boiling water and freely soluble in glycerol. – Identification (A) Reaction
(B) Reaction
(C) Reaction
A. Solution S is faintly acid
B. Reaction of Potassium
C. Reaction for Phosphates
A. Reaction of Potassium B. Reaction for Phosphates
pH (4% w/v solution in water) 4.2-4.5 – Sulphate NMT 50 ppm.
– Ammonium NMT10 ppm.
– Arsenic NMT 2 ppm. NMT 3 ppm Sodium NMT 1000 ppm – Iron NMT 10 ppm – Sulphate NMT 300 ppm – Chloride NMT 200 ppm – Lead – NMT 5 ppm Heavy Metal – NMT 20 ppm Fluoride – NMT 10 ppm Reducing Substances The colour of permanganate is not completely discharged – Residual Solvents – Meets the requirement Organic volatile impurities – Meets the requirement Insoluble substances – Max. 0.2% Loss on Drying at 1050C Max. 2.0% Max. 1.0% Assay Na2B4O7,10H2O 98.0 % – 100.5 98.0 % to 100.5 % Potassium Sulphate
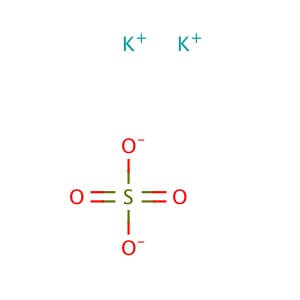
CAS NO: 7778-80-5
MOLECULAR FORMULA : K2SO4
MOLECULAR WEIGHT : 174.26
SPECIALITY FINE CHEMICALS (AR / LR / ACR /GR / IP / BP / USP / FSSAI )Potassium Sulphate Crystals
CAS NO: 7778-80-5
MOLECULAR FORMULA : K2SO4
MOLECULAR WEIGHT : 174.26
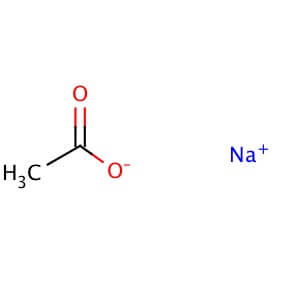
SPECIALITY FINE CHEMICALS (AR / LR / ACR /GR / IP / BP / USP / FSSAI )Sodium Acetate Anhydrous
CAS NO: 127-09-3
MOLECULAR FORMULA : C2H3NaO2
MOLECULAR WEIGHT: 82.0
Description Synonyms Acetic acid sodium salt Product Information CAS number 127-09-3 Hill Formula C₂H₃NaO₂ Chemical formula CH₃COONa Molar Mass 82.03 g/mol HS Code 2915 29 00 Structure formula Image Quality Level MQ100 Applications Application Sodium acetate anhydrous 99.99 Suprapur®. CAS No. 127-09-3, EC Number2048238. Physicochemical Information Boiling point >400 °C (decomposition) Density 1.528 g/cm3 Flash point >250 °C Ignition temperature 607 °C Melting Point 324 °C (decomposition) pH value 7.5 – 9.2 (30 g/l, H₂O, 20 °C) Solubility 365 g/l Toxicological Information LD 50 oral LD50 Rat 3530 mg/kg LD 50 dermal LD50 Rabbit > 10000 mg/kg Safety Information according to GHS RTECS AJ4300010 Storage class 10 – 13 Other liquids and solids WGK WGK 1 slightly hazardous to water Storage and Shipping Information Storage Store at +2°C to +30°C. Specifications Purity (metallic) ≥ 99.99 % Assay (perchloric acid titration) ≥ 99 % Chloride (Cl) ≤ 5 ppm Phosphate (PO₄) ≤ 5 ppm Sulfate (SO₄) ≤ 20 ppm Al (Aluminium) ≤ 0.05 ppm Ba (Barium) ≤ 5.0 ppm Bi (Bismuth) ≤ 0.01 ppm Ca (Calcium) ≤ 0.10 ppm Cd (Cadmium) ≤ 0.005 ppm Ce (Cerium) ≤ 0.005 ppm Co (Cobalt) ≤ 0.005 ppm Cr (Chromium) ≤ 0.010 ppm Cs (Cesium) ≤ 2.0 ppm Cu (Copper) ≤ 0.005 ppm Fe (Iron) ≤ 0.05 ppm K (Potassium) ≤ 5 ppm La (Lanthanum) ≤ 0.005 ppm Mg (Magnesium) ≤ 0.10 ppm Mn (Manganese) ≤ 0.050 ppm Ni (Nickel) ≤ 0.010 ppm Pb (Lead) ≤ 0.005 ppm Rb (Rubidium) ≤ 0.5 ppm Sc (Scandium) ≤ 0.005 ppm Sm (Samarium) ≤ 0.005 ppm Sr (Strontium) ≤ 0.10 ppm Tl (Thallium) ≤ 0.005 ppm Y (Yttrium) ≤ 0.005 ppm Zn (Zinc) ≤ 0.010 ppm Sodium Acetate Trihydrate
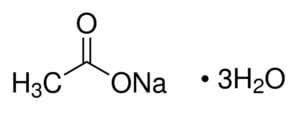
CAS NO : 6131-90-4
MOLECULAR FORMULA :C2H9NaO5
MOLECULAR WEIGHT : 136.08
Product Information CAS number 6131-90-4 Grade Ph Eur,BP,JP,USP,FCC,E 262 Hill Formula C₂H₃NaO₂ * 3 H₂O Chemical formula CH₃COONa * 3 H₂O Molar Mass 136.08 g/mol HS Code 2915 29 00 Structure formula Image Quality Level MQ600 Physicochemical Information Density 1.45 g/cm3 Ignition temperature 607 °C Melting Point 57.9 °C pH value 8.5 – 10 (408 g/l, H₂O, 25 °C) Bulk density 900 kg/m3 Solubility 613 g/l Toxicological Information LD 50 oral LD50 Rat 3530 mg/kg LD 50 dermal LD50 Rabbit > 10000 mg/kg Safety Information according to GHS RTECS AJ4580000 Storage class 10 – 13 Other liquids and solids WGK WGK 1 slightly hazardous to water Disposal 14 Inorganic salts: Container I. Neutral solutions of the these salts: Container D. Before placing in Container D, check the pH with pH-Universal indicator strips (Item No. 109535). Storage and Shipping Information Storage Store at +2°C to +25°C. Transport Information Declaration (railroad and road) ADR, RID Kein Gefahrgut Declaration (transport by air) IATA-DGR No Dangerous Good Declaration (transport by sea) IMDG-Code No Dangerous Good Specifications Assay (perchloric acid titration, calculated on dried substance) 99.5 – 101.0 % Identity passes test Appearance of solution passes test In water insoluble matter ≤ 0.03 % Alkalinity ≤ 0.05 % Acidity or alkalinity passes test pH-value (1 %, water) 8.0 – 9.5 pH-value (5 %; water) 7.5 – 9.0 Chloride (Cl) ≤ 0.005 % Sulfate (SO₄) ≤ 0.003 % Heavy metals (as Pb) ≤ 0.0006 % Al (Aluminium) ≤ 0.00002 % As (Arsenic) ≤ 0.0001 % Ca (Calcium) ≤ 0.002 % Fe (Iron) ≤ 0.0010 % Hg (Mercury) ≤ 0.0001 % K (Potassium) ≤ 0.005 % Mg (Magnesium) ≤ 0.001 % Pb (Lead) ≤ 0.0002 % Ca + Mg (calcium and magnesium) passes test free acetic acid ≤ 0.4 % Other residual solvents (ICH Q3C) excluded by manufacturing process formic acid, formate and other oxidizable impurities ≤ 0.1000 % Substances reducing potassium permanganate (as HCOOH) passes test Loss on drying (130 °C) 39.0 – 40.5 % Sodium Benzoate
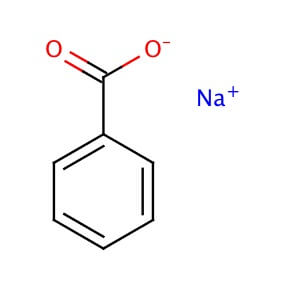
CAS NO: 532-32-1
MOLECULAR FORMULA : C7H5O2•Na
MOLECULAR WEIGHT: 144.10
Product Information Grade Ph Eur,BP,NF,FCC,E 211 Hill Formula C₇H₅NaO₂ Chemical formula C₆H₅COONa Molar Mass 144.10 g/mol HS Code 2916 31 00 Quality Level MQ500 Physicochemical Information Density 1.50 g/cm3 (20 °C) Ignition temperature >500 °C Melting Point 436 °C pH value 8 (100 g/l, H₂O, 20 °C) Bulk density 350 kg/m3 Solubility 556 g/l Toxicological Information LD 50 oral LD50 Rat 3140 mg/kg Safety Information according to GHS Hazard Pictogram(s) Hazard Statement(s) H319: Causes serious eye irritation. Precautionary Statement(s) P264: Wash skin thoroughly after handling. P280: Wear eye protection/ face protection. P305 + P351 + P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P337 + P313: If eye irritation persists: Get medical advice/ attention. Signal Word Warning RTECS DH6650000 Storage class 10 – 13 Other liquids and solids WGK WGK 1 slightly hazardous to water Disposal 3 Relatively unreactive organic reagents should be collected in container A. If halogenated, they should be collected in container B. For solid residues use container C. Storage and Shipping Information Storage Store at +2°C to +25°C. Transport Information Declaration (railroad and road) ADR, RID Kein Gefahrgut Declaration (transport by air) IATA-DGR No Dangerous Good Declaration (transport by sea) IMDG-Code No Dangerous Good Specifications Assay (acidimetric, calc. on anhydrous substance) 99.0 – 100.5 % Assay (HPLC, calc. on anhydrous substance) 99.0 – 101.0 % Identity (IR-spectrum) passes test Identity (HPLC) passes test Identity (wet chemistry) passes test Appearance of solution (100 g/l, water) clear and not more intense in color than reference solution Y6 Acidity or alkalinity passes test Chloride (Cl) ≤ 200 ppm Total chlorine ≤ 300 ppm As (Arsenic) ≤ 3 ppm Hg (Mercury) ≤ 1 ppm Pb (Lead) ≤ 2 ppm Residual solvents (ICH Q3C) excluded by production process Multinuclear acids passes test Oxidizable matter passes test Water (according to Karl Fischer) ≤ 1.5 % Sodium Bi Carbonate
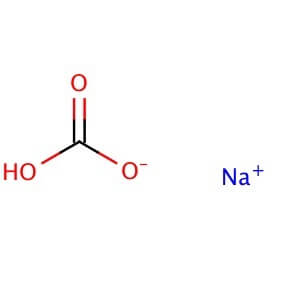
CAS NO: 144-58-8
MOLECULAR FORMULA: NaHCO3
MOLECULAR WEIGHT: 84.01
Alternate Names: Sodium hydrogen carbonate Application: Sodium bicarbonate is a commonly used laboratory pH neutralizer that reacts with acids and bases Purity: ≥99% Appearance : Powder Physical State : Solid Solubility : Soluble in water (50 mg/ml). Insoluble in ethanol. Storage : Store at room temperature Melting Point : 300° C Density : 2.16 g/cm3 at 20° C Refractive Index : n20D 1.50 Ki Data : CA IV: Ki= 6.6 mM (human); CA I: Ki= 12 mM (human); CA IX: Ki= 13 mM (human); Astrosclerin-3: Ki= 22.8 mM (human); CA II: Ki= 85 mM (human) pK Values : pKa: 6.37, 10.25 in carbonic acid(25°C) Sodium BiSulphite
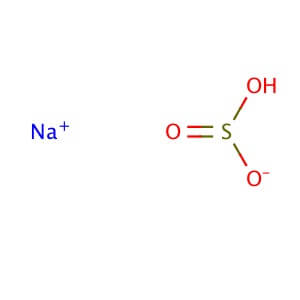
CAS NO: 7631-90-5
MOLECULAR FORMULA: NaHSO3
MOLECULAR WEIGHT: 104.06
Molecular Formula: NaHSO3 Molecular Weight: 104.06 Alternate Names: Sodium hydrogensulfite, mixture of NaHSO3 and Na2S2O5 Application: Sodium bisulfite, mixture of NaHSO3 and Na2S2O5 is an antioxidant and antimicrobial agent for DNA methylation studies Appearance : Powder or crystalline or crystalline powder Physical State : Solid Solubility : Soluble in hot water (very), cold water (very), and alchol (slightly). Storage : Store at room temperature Melting Point : 300° C Density : 1.48 g/cm3
Showing 19–36 of 56 items