Description
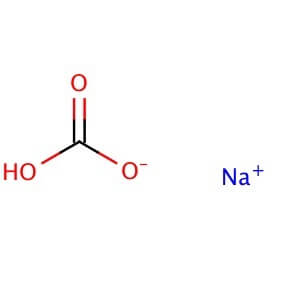
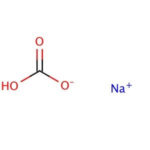
Sodium bicarbonate is a commonly used laboratory chemical. The compound can react with both acids and bases, therefore it can be used as a neutralizer. When reacting with bases the compound forms carbonates. Sodium bicarbonate may be used to remove or wash acidic impurities from a crude liquid to yield a purer sample. Sodium bicarbonate reacts with the carboxyl groups to give a fast-forming and effervescent CO2 formation which has been used to test for the presence of carboxylic groups in proteins. Sodium bicarbonate has been observed to significantly decrease serum potassium levels as well as increase blood pH and bicarbonate concentration. The compound has been recorded to decrease the duration of peripheral nerve block in rats when added to Lidocaine.

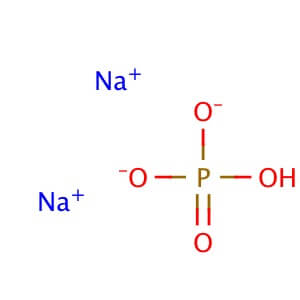
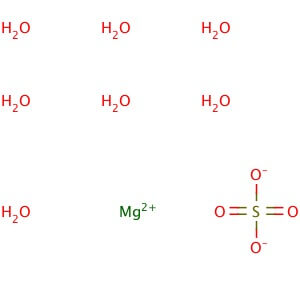
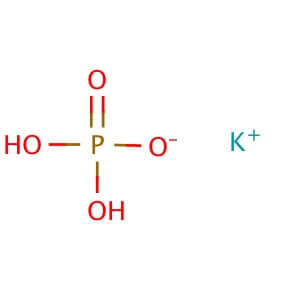
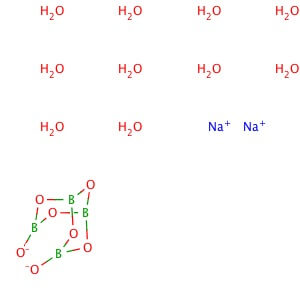

Reviews
There are no reviews yet.