Description
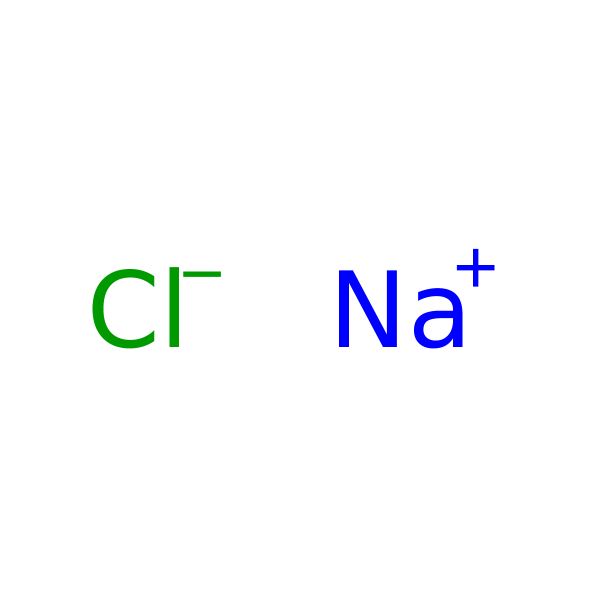
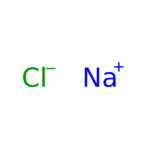
Sodium Chloride (NaCl) is widely used in biochemistry and molecular biology applications. Sodium Chloride is found in nature, in all body tissue, and is considered an essential nutrient. Sodium Chloride is used in a wide variety of biochemical applications including creating density gradients, regulating biological functions, and as a diluent to increase ionic strength in buffers or culture media. Sodium Chloride is a component of phosphate buffered saline (PBS) and SSC buffer. Sodium Chloride has also been used for precipitation of DNA from SDS-containing samples and in the removal of small nucleic acid fragments from plasmid DNA preparations. The use of Sodium Chloride for isolation of DNA from mammalian cells has been described. Recent studies provide evidence that a high amount of Sodium Chloride reduces production of nitric oxide. Sodium Chloride is also known as table salt, and Halite.

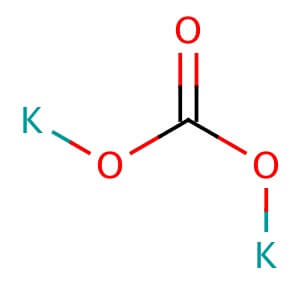
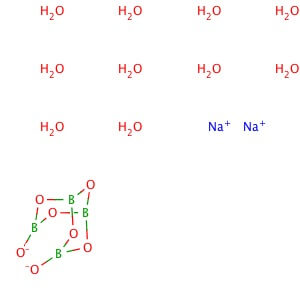
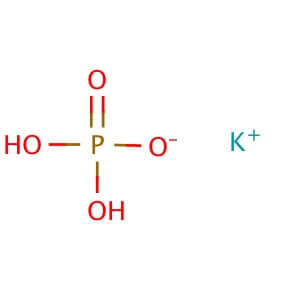
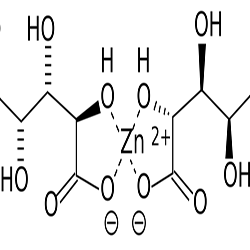

Reviews
There are no reviews yet.