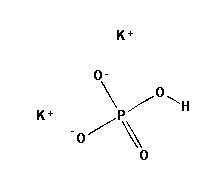
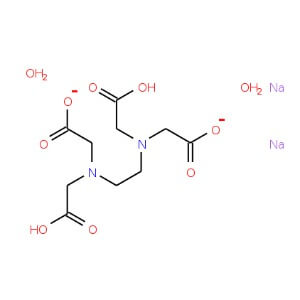
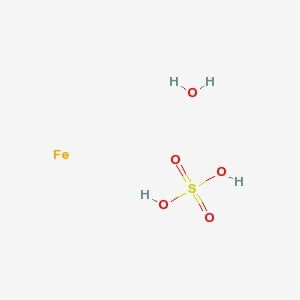
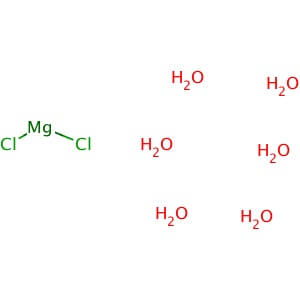
Dibasic Potassium Phosphate
CAS NO:7758-11-4
MOLECULAR FORMULA : K2HPO4
MOLECULAR WEIGHT : 174.18
SPECIFICATION BP USP Description White or almost white powder or colorless crystals, very hygroscopic. White or almost white powder or colorless crystals, very hygroscopic. Solubility Very soluble in water, very slightly soluble in ethanol (96 per cent). – Appearance of solution Solution is clear & Colorless – Identification (A) ) Reaction
(B) Reaction
(C) Reaction
A. Solution S (see Tests) is slightly alkaline B. Solution S gives reaction of phosphate
C. Solution S gives reaction of potassium
A. Solution S gives reaction of phosphate B. Solution S gives reaction of potassium
Reducing substances The solution remains faintly pink. – Heavy Metals – NMT 0.001 % Limit of Fluoride – NMT 0.001 % pH – 8.5-9.6 Monopotassium phosphate This ratio is not greater than 0.025. – Chlorides NMT200 ppm – Sulphates NMT 0.1 % – Arsenic NMT 2 ppm – Sodium NMT 0.1 % A solution tested on a platinum wire imparts no pronounced yellow color to a nonluminous flame. Limit of Monobasic OR tribasic salt – NMT 0.001 % Iron NMT10 ppm NMT 30 ppm Residual solvents – Meets the requirement Loss on Drying(at 125-130˚C) NMT 2.0 % NMT 1.0 % Assay (dried substance). 98.0 % to 101.0 % 98.0 % to 100.5 % Disodium Edetate
CAS NO: 6381-92-6
MOLECULAR FORMULA :- C10H14N2Na2O82H2O
MOLECULAR WEIGHT :- 372.24 g/ mol
SPECIFICATION IP BP USP Description A white ,crystalline powder, Odourless White or almost white, crystalline powder. White or almost white, crystalline powder. Solubility – Soluble in water, practically insoluble in ethanol (96 %) – Identification A. IR
B. Reaction
C. Reaction
D. Reaction
A. Must comply with the disodium edetate RS
B. No precipitate is produced.
C. No precipitate is produced.
D. It gives reaction of sodium salt.
A. Must comply with the disodium edetate RS
B. No precipitate is produced.
C. No precipitate is produced.
D. It gives reaction of sodium salt.
A. Must comply with the disodium edetate RS
B. The red color is discharge, leaving a yellowish solution
C. It gives reaction of sodium salt.
Appearance of solution 5 % w/v solution in carbon dioxide free water is Clear & colorless Clear & colorless – pH 4.0-5.5 ( 5 % w/v solution ) 4.0-5.5 ( 5 % w/v solution ) 4.0-6.0 Loss on Drying – – 8.7 %-11.4 % Calcium – – No precipitate is formed. Impurity A Limit of nitrilotriacetic acid(USP)
NMT 0.1 % NMT 0.1 % NMT 0.1 % Heavy metals NMT 20 ppm – NMT 50 ppm Iron NMT 80 ppm NMT 80 ppm – Assay 98.5 %-101.0 % 98.5 %-101.0 % 99.0 %-101.0 %(on Dried basis) Ferrous Sulfate Heptahydrate
CAS NO: 7782-63-0
MOLECULAR FORMULA :- FeSO4.7H2O
MOLECULAR WEIGHT :- 278.02 g/mol
SPECIFICATION IP BP USP Description Bluish green crystals or a light green, crystalline powder, odourless.Efforescent in air. On exposure To moist air, the crystals rapidly oxidize and become brown. Bluish green crystals or a light green, crystalline powder,efforescent in air. Bluish green crystals or a light green, crystalline powder, odourless.Efforescent in air. Solubility – Freely soluble in water, very soluble in boiling water, practically insoluble in ethanol (96 %) Ferrous sulfate heptahydrate is oxidized in moist air, becoming brown. – pH 3.0-4.0 ( 5 % w/v Solution) 3.0-4.0 – Appearance of Solution The solution is not more opalescent than opalescent Standard. – – Identification (A) Reaction
(B) Reaction
(C) Assay
A. It gives reaction of sulfates B. It gives reaction of Ferrous Salt
A. It gives reaction of sulfates B. It gives reaction of Irons
C. It complies with limit of assay.
A. It gives reaction of sulfates B. It gives reaction of Ferrous Salt
Chloride NMT 250 ppm NMT 200 ppm – Basic sulphate Producing solution that is not more than turbid. – – Arsenic NMT 2 ppm – NMT 3 ppm Lead NMT 50 ppm – NMT 10 ppm Mercury – – It meets the requirements of the test for mercury Chromium – NMT 50 ppm – Copper NMT 50 ppm NMT 50 ppm – Ferric ions NMT 0.5 % NMT 0.3 % – Manganese NMT 0.1 % NMT 0.1 % – Nickel – NMT 50 ppm – Zinc NMT 500 ppm NMT 50 ppm – Organic volatile impurities – – Meets the requirements. Residual Solvents – – Meets the requirements. Assay 98.0 % – 105.0 % 98.0 % – 105.0 % 99.5% -104.5 % Magnesium Chloride Hexahydrate
CAS NO: 7791-18-6
MOLECULAR FORMULA: MgCl2•6H2O
MOLECULAR WEIGHT: 203.30
Application: Magnesium Chloride, Hexahydrate is a source of magnesium ion and a co-foactor for many enzymes Purity: >99% Molecular Weight: 203.3 Molecular Formula: MgCl2•6H2O Description: Magnesium Chloride, Hexahydrate is widely used as a source of magnesium ion in chemistry and molecular biology applications. In biological systems, magnesium is a co-factor for many enzymes including deoxyribonuclease (DNase) and various restriction enzymes. Also plays a role in cell membrane integrity, muscle cell physiology, cardiovascular and muscular activity, and nucleic acid structure. Magnesium chloride solution is a favorable choice as an elution buffer for antibody affinity column purifications; it is much milder on most antigens than acid elution, allowing reuse of the antigen column. Also an essential cofactor for the DNA polymerase in polymerase chain reaction (PCR) amplification. Physical State : Solid Solubility : Soluble in water (20 mg/ml), and alcohol. Storage : Store at room temperature Melting Point : 116-118° C (dec.) Density : 1.57 g/cm3 at 20° C Magnesium Sulfate Heptahydrate
CAS NO: 10034-99-8
MOLECULAR FORMULA :- MgSO4.7H20
MOLECULAR WEIGHT :- 246.50 g/mol
SPECIFICATION IP BP USP Description Colorless crystals or white, Crystalline powder. White or almost white, crystalline powder or brilliant , colorless crystals. Colorless crystals or white, Crystalline powder Solubility – Freely Soluble in water , very soluble in boiling water and practically insoluble in ethanol (96 % )
Identification (A) Reaction
(B) Reaction
A : Reactions of Sulphates B : Reactions of Magnesium salts
A: It gives reaction of Sulphates. B :It gives reaction of magnesium
A: It gives reaction of Sulphates. B : It gives reaction of magnesium
pH of a 5% solution at 250C – – 5.0 – 9.2 Appearance of solution Dissolve 5 g in sufficient carbon dioxide free water to produced 50 ml (Solution A) is clear and colorless. Dissolve 5 g in sufficient carbon dioxide free water to produced 50 ml (Solution S) is clear and colorless. – Acidity or alkalinity NMT 0.2 ml of either 0.01 M hydrochloric acid or 0.01 M sodium hydroxide is required to change the color of solution. NMT.0.2 ml of 0.01 M hydrochloric or 0.01 M sodium hydroxide is required to change the color of indicator. – Arsenic NMT 2 ppm.
NMT 2 ppm – Heavy metals NMT 10 ppm.
– NMT 10 ppm Selenium – NMT 30 ppm Iron NMT 200 ppm. NMT 20 ppm NMT 20 ppm Chloride NMT. 300 ppm NMT. 300 ppm
NMT. 140 ppm Organic Volatile impurities – Meets the requirement Residual Solvents – Meets the requirement Loss on drying 48.0 to 52.0 %
48.0 % – 52.0 % NMT 2.0 % Loss on Ignition – – 40.0%-52.0% Assay (on dried basis) 99.0 % to 100.5 % 99.0 % – 100.5 % 99.0%- 100.5% Potassium Acetate
CAS NO: 127-08-2
MOLECULAR FORMULA :- C2H3KO2
MOLECULAR WEIGHT :- 98.14 g/mol
SPECIFICATION BP USP Description White or almost white, crystalline powder or colorless cryastals,deliquescent. White or almost white, crystalline powder or colorless cryastals,deliquescent. Solubility Very soluble in water, freely soluble in ethanol (96 %) – Identification A. Reaction
B. Reaction
A. Reaction of acetates.
B. Reaction of potassium
A. Reaction of acetates.
B. Reaction of potassium
Appearance of solution Clear & Colorless – pH 7.5-9.0 7.5-8.5 Heavy metals – NMT 20 ppm Limit of Sodium – NMT 0.03 % Reducing substances The solution remains pink. – Chloride NMT 200 ppm – Sulfates NMT 200 ppm – Aluminum NMT 1 ppm – Iron NMT 20 ppm – sodium NMT 0.5 % – Loss on drying NMT 3.0 % NMT 1.0 % Residual Solvents – Meets the requirements Assay 99.0 %-101.0 % 99.0%-100.5 %
Showing 7–12 of 37 items