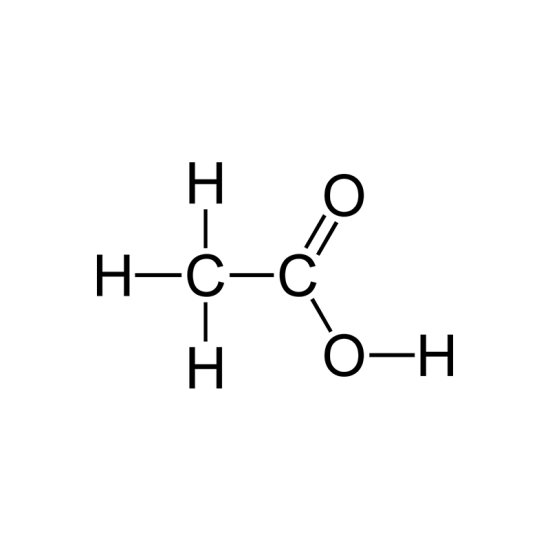
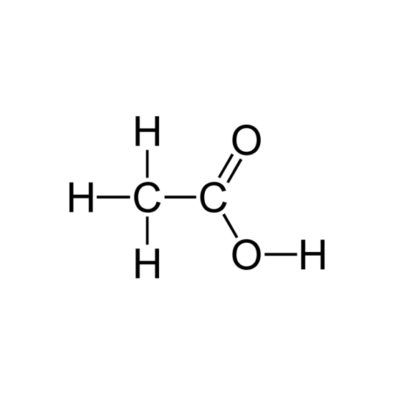
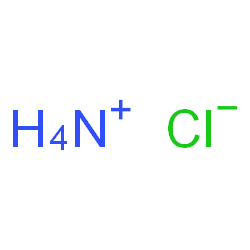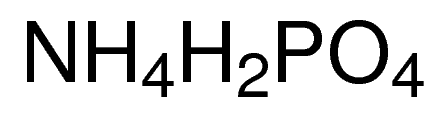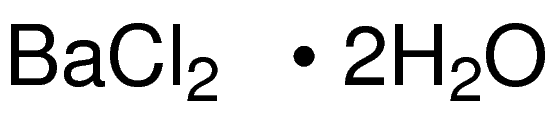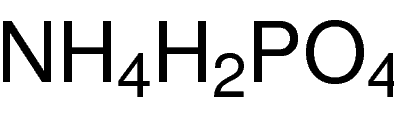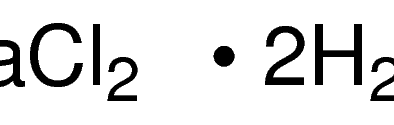Acetic Acid Glacial
CAS NO: 64-19-7
MOLECULAR FORMULA : C2H4O2
MOLECULAR WEIGHT : 60.05
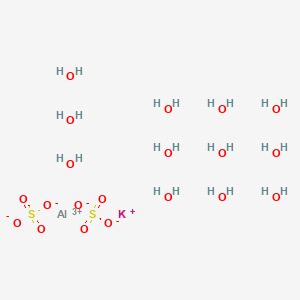
SPECIALITY FINE CHEMICALS (AR / LR / ACR /GR / IP / BP / USP / FSSAI )Aluminium Potassium Sulphate
CAS NO: 7784-24-9
MOLECULAR FORMULA : AlK(SO4)2•12H2O
MOLECULAR WEIGHT : 474.39
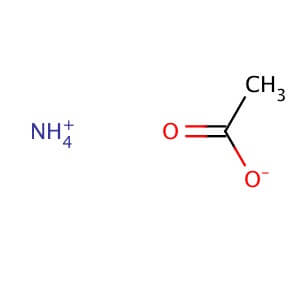
SPECIALITY FINE CHEMICALS (AR / LR / ACR /GR / IP / BP / USP / FSSAI )Ammonium Acetate
CAS NO: 631-61-8
MOLECULAR FORMULA :- CH3COONH4
MOLECULAR WEIGHT :- 77.08
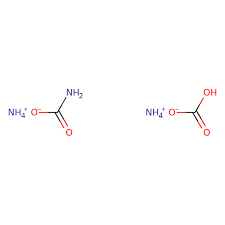
SPECIFICATION LR AR ACS FCC Insoluble Matter — 0.001% 0.005% pH of a 5% solution at 250C 6.0 -7.5 6.5 – 7.5 6.7 – 7.3 Chloride (Cl) 0.002% 0.0005% 0.0005% Residue After Ignition — — 0.01% Sulphate (SO4) 0.01% 0.002% 0.001% Heavy Metals (as Pb) 0.0005% 0.0001% 0.0005% Iron (Fe) 0.001% 0.0001% 0.0005% Phosphate (PO4) — 0.0005% — Calcium (Ca) — 0.0005% — Copper (Cu) — 0.0001% — Magnesium (Mg) — 0.0001% — Potassium (K) 0.002% 0.001% — Sodium (Na) — 0.001% — Ammonium Carbonate
CAS NO: 8000-73-5
MOLECULAR FORMULA : C2H11N3O5
MOLECULAR WEIGHT : 157.13 g/mol
SPECIALITY FINE CHEMICALS (AR / LR / ACR /GR / IP / BP / USP / FSSAI )Ammonium Chloride
CAS NO: 12125-02-9
MOLECULAR FORMULA : NH4Cl
MOLECULAR WEIGHT : 53.49
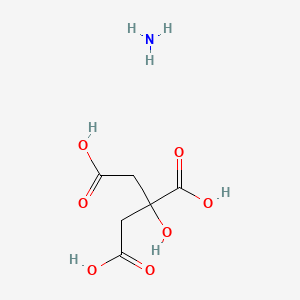
SPECIALITY FINE CHEMICALS (AR / LR / ACR /GR / IP / BP / USP / FSSAI )Ammonium Citrate
CAS NO: 3012-65-5
MOLECULAR FORMULA : C6H14N2O7
MOLECULAR WEIGHT : 226.18
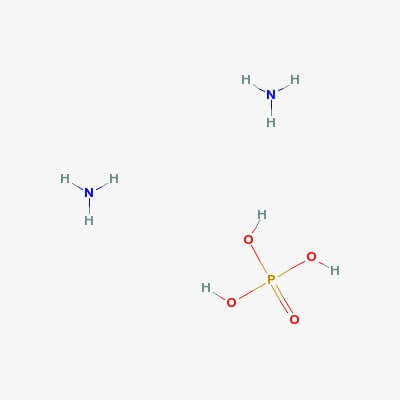
SPECIALITY FINE CHEMICALS (AR / LR / ACR /GR / IP / BP / USP / FSSAI )Ammonium Phosphate Dibasic
CAS NO: 7783-28-0
Molecular Formula: HPO4·(NH4)2
Molecular Weight: 132.06
Description Replaces AX1355-1; AX1355 Synonyms Ammonium biphosphate, Ammonium phosphate dibasic, Diammonium hydrogen phosphate, Fyrex Description di-Ammonium hydrogen phosphate Product Information CAS number 7783-28-0 EC number 231-987-8 Grade ACS,Reag. Ph Eur Hill Formula H₉N₂O₄P Chemical formula (NH₄)₂HPO₄ Molar Mass 132.06 g/mol HS Code 3105 30 00 Quality Level MQ300 Physicochemical Information Density 1.620 g/cm3 Melting Point 155 °C (decomposition) pH value 7.5 – 9.0 (132.1 g/l, H₂O, 25 °C) Vapor pressure 0.076 Pa (20 °C) Bulk density 800 – 1000 kg/m3 Solubility 690 g/l Toxicological Information LD 50 oral LD50 Rat 6500 mg/kg LD 50 dermal LD50 Rabbit > 7950 mg/kg Safety Information according to GHS Storage class 10 – 13 Other liquids and solids WGK WGK 1 slightly hazardous to water Disposal 14 Inorganic salts: Container I. Neutral solutions of the these salts: Container D. Before placing in Container D, check the pH with pH-Universal indicator strips (Item No. 109535). Storage and Shipping Information Storage Store at +2°C to +30°C. Transport Information Declaration (railroad and road) ADR, RID Kein Gefahrgut Declaration (transport by air) IATA-DGR No Dangerous Good Declaration (transport by sea) IMDG-Code No Dangerous Good Specifications Assay (acidimetric) ≥ 99.0 % Insoluble matter ≤ 0.005 % pH-value (5 %; water; 25 °C) 7.8 – 8.1 pH-value (20 %; water) about 8 Chloride (Cl) ≤ 0.0005 % Nitrate (NO₃) ≤ 0.001 % Sulfate ≤ 0.004 % Heavy metals (as Pb) ≤ 0.0005 % Ca (Calcium) ≤ 0.001 % Fe (Iron) ≤ 0.001 % K (Potassium) ≤ 0.001 % Mg (Magnesium) ≤ 0.0005 % Na (Sodium) ≤ 0.001 % Corresponds to ACS, Reag. Ph Eur Ammonium Phosphate Monobasic
CAS NO: 7722-76-1
MOLECULAR FORMULA : NH4H2PO4
MOLECULAR WEIGHT : 115.03
SPECIALITY FINE CHEMICALS (AR / LR / ACR /GR / IP / BP / USP / FSSAI )
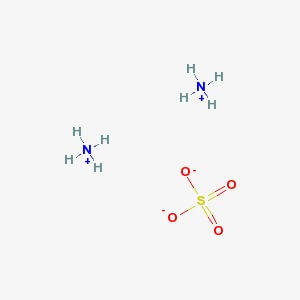
Ammonium Sulphate
CAS NO: 7783-20-2
MOLECULAR FORMULA : (NH4)2SO4
MOLECULAR WEIGHT : 132.1
SPECIALITY FINE CHEMICALS (AR / LR / ACR /GR / IP / BP / USP / FSSAI )Barium Chloride Dihydrate
CAS NO: 10326-27-9
MOLECULAR FORMULA: BaCl2•2H2O
MOLECULAR WEIGHT: 244.26
SPECIALITY FINE CHEMICALS (AR / LR / ACR /GR / IP / BP / USP / FSSAI )
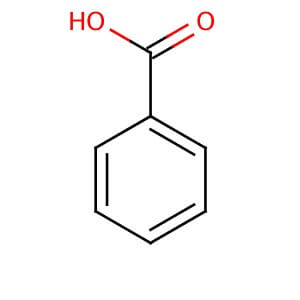
Benzoic Acid
CAS NO: 65-85-0
MOLECULAR FORMULA: C7H6O2
MOLECULAR WEIGHT: 122.12
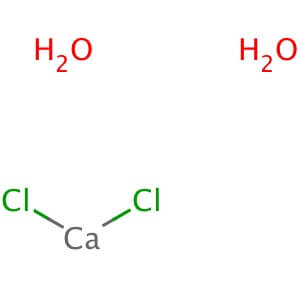
Application: Benzoic acid is a simple organic compound employed as a preservative Description Benzoic acid is a weak acid that is a precursor for the synthesis of many important organic compounds. L. monocytogens has displayed an acid adaption to the presence of Benzoic acid with a decrease in total lipid phosphorus. Appearance : Powder Physical State : Solid Solubility : Soluble in ethanol (100 mg/ml), water (3.5 mg/ml at 25° C), chloroform (15 mg/ml), methanol (71.5 mg/ml), and hexane (0.9 mg/ml). Storage : Store at room temperature Melting Point : 121-125° C (lit.) Boiling Point : 249° C (lit.) Density : 1.32 g/cm3 at 20° C Refractive Index : n20D 1.56 (Predicted) IC50 : Protobothrops flavoviridis venom-induced hemorrhagic lesion formation compound: IC50 = 200 nM (mouse); DAAO: IC50 = 46.9 µM (human) Ki Data : DAAO: Ki= 7 µM (human); Solute carrier family 22 member 20: Ki= 13.8 µM (mouse); Solute carrier family 22 member 6: Ki= 251.19 µM (mouse) pK Values : pKa: 4.19 (25° C) Calcium Chloride Dihydrate
CAS NO: 10035-04-8
MOLECULAR FORMULA :- CaCl2.2H2O
MOLECULAR WEIGHT :- 147.01 g/mol
SPECIFICATION IP BP USP Description A White, Crystalline powder or fragments or granules, odorless, hygroscopic. White or almost white, crystalline powder, hygroscopic. White or almost white, crystalline powder, hygroscopic. Solubility – Freely soluble in water and soluble in ethanol (96 %) – Appearance of solution Clear and not more intensely colored than reference solution YS6. 10 % solution is clear and not more intensely colored than reference solution Y6. – Identification (A) Reaction Of Calcium
(B) Reaction of Chlorides
(C) Assay
A. Reactions of Calcium salts B. Reactions of Chlorides
A. 10% solution gives reaction of chloride B. It gives reaction of calcium
C. It complies with the limit test of assay
A. Reactions of Calcium salts B. Reactions of Chlorides
Acidity or Alkalinity To 10 ml of freshly prepared 10 % w/v solution add 2 drops off phenolphthalein solution. Titrate with 0.01 M hydrochloric acid or 0.01 M sodium hydroxide. If the solution is red , than not more 0.2 ml of 0.01 M HCL need to discharge the color and if the solution is colorless, not more than 0.2 ml NaOH is required to turn it red. – pH – – 4.5 – 9.2 Arsenic NMT 3 ppm. – – Barium After not less than 15 min. the solution is not more opalescent than a mixture of 10 ml of solution and 1 ml of distilled water. After 15 min, Any opalescent in the solution is not more intense than that in a mixture of 1 ml of distilled water and 1.0 gm of sample. – Aluminum &Phosphate (IP)
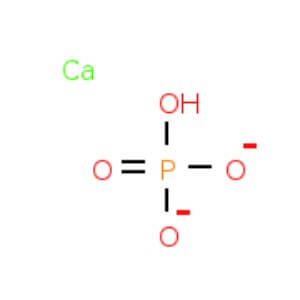
No turbidity or precipitate is produced. No turbidity or precipitate is formed. NMT 1 ppm Iron , Aluminum &Phosphate – – No turbidity or precipitate is formed. Heavy metals NMT10 ppm – NMT10 ppm Iron NMT 20 ppm. NMT 10 ppm – Magnesium and Alkali salts NMT 1.0 % NMT 0.5 % NMT 1.0 % Sulphate NMT 300 ppm NMT 300 ppm – Organic Volatile impurities – – Meets the requirements Assay 97.0 % to 103.0 % 97.0 % – 103.0 % 99.0% – 107.0% Calcium Phosphate Di Basic
CAS NO: 7757-93-9
Molecular Formula: CaHPO4
Molecular Weight: 136.06
Description Synonyms Calcium orthophosphate, Calcium phosphate dibasic Description Calcium hydrogen phosphate Product Information CAS number 7757-93-9 EC number 231-826-1 Grade Ph Eur,BP,USP,FCC,E 341 (ii) Hill Formula CaHO₄P Chemical formula CaHPO₄ Molar Mass 136.06 g/mol HS Code 2835 25 00 Quality Level MQ500 Physicochemical Information Density 2.89 g/cm3 (20 °C) Melting Point 370 °C (decomposition) pH value 7 (10 g/l, H₂O, 20 °C) suspension Bulk density 900 kg/m3 Solubility 0.1 g/l Toxicological Information LD 50 oral LD50 Rat 10000 mg/kg LD 50 dermal LD50 Rabbit > 7940 mg/kg Safety Information according to GHS RTECS TB8528000 Storage class 10 – 13 Other liquids and solids WGK WGK 1 slightly hazardous to water Disposal 14 Inorganic salts: Container I. Neutral solutions of the these salts: Container D. Before placing in Container D, check the pH with pH-Universal indicator strips Storage and Shipping Information Storage Store at +2°C to +30°C. Transport Information Declaration (railroad and road) ADR, RID Kein Gefahrgut Declaration (transport by air) IATA- DGR No Dangerous Good Declaration (transport by sea) IMDG- Code No Dangerous Good Specifications Identity passes test Assay (complexometric) 97.5 – 102.5 % Assay (complexometric; calculated on dried substance) 98.0 – 102.0 % Substances insoluble in hydrochloric acid ≤ 0.2 % Carbonate (as CO₂) passes test Chloride (Cl) ≤ 0.03 % Fluoride (F) ≤ 0.0050 % Sulfate (SO₄) ≤ 0.5 % Al (Aluminium) ≤ 0.0200 % As (Arsenic) ≤ 0.0001 % Ba (Barium) passes test Cd (Cadmium)* ≤ 0.0001 Fe (Iron) ≤ 0.0400 % Hg (Mercury) ≤ 0.0001 % Ni (Nickel)* ≤ 0.0010 Pb (Lead)* ≤ 0.0001 Sr (Strontium) ≤ 0.03 % V (Vanadium)* ≤ 0.0010 % Residual solvents (ICH Q3C) excluded by production process Loss on ignition (800 °C) 7.0 – 8.5 % Loss on drying ≤ 2.0 % Particle size (< 63 µm) about 99 % Elemental impurity specifications have been set considering ICH Q3D (Guideline for Elemental Impurities). Class 1-3 elements are not likely to be present above the ICH Q3D option 1 limit, unless specified and indicated (*). Corresponds to Ph.Eur.,BP,USP,FCC,E341 (ii) Conforms to the purity criteria on food additives, except for use as additive in infant and young child nutrition, according to the current European Commission Regulation. Cupric Sulphate
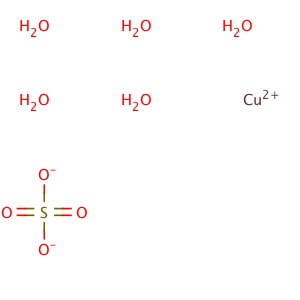
CAS NO: 7758-99-8
MOLECULAR FORMULA : CuH10O9S
MOLECULAR WEIGHT : 249.69
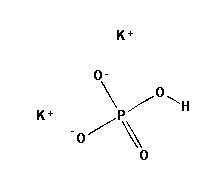
SPECIALITY FINE CHEMICALS (AR / LR / ACR /GR / IP / BP / USP / FSSAI )Dibasic Potassium Phosphate
CAS NO:7758-11-4
MOLECULAR FORMULA : K2HPO4
MOLECULAR WEIGHT : 174.18
SPECIFICATION BP USP Description White or almost white powder or colorless crystals, very hygroscopic. White or almost white powder or colorless crystals, very hygroscopic. Solubility Very soluble in water, very slightly soluble in ethanol (96 per cent). – Appearance of solution Solution is clear & Colorless – Identification (A) ) Reaction
(B) Reaction
(C) Reaction
A. Solution S (see Tests) is slightly alkaline B. Solution S gives reaction of phosphate
C. Solution S gives reaction of potassium
A. Solution S gives reaction of phosphate B. Solution S gives reaction of potassium
Reducing substances The solution remains faintly pink. – Heavy Metals – NMT 0.001 % Limit of Fluoride – NMT 0.001 % pH – 8.5-9.6 Monopotassium phosphate This ratio is not greater than 0.025. – Chlorides NMT200 ppm – Sulphates NMT 0.1 % – Arsenic NMT 2 ppm – Sodium NMT 0.1 % A solution tested on a platinum wire imparts no pronounced yellow color to a nonluminous flame. Limit of Monobasic OR tribasic salt – NMT 0.001 % Iron NMT10 ppm NMT 30 ppm Residual solvents – Meets the requirement Loss on Drying(at 125-130˚C) NMT 2.0 % NMT 1.0 % Assay (dried substance). 98.0 % to 101.0 % 98.0 % to 100.5 % Disodium Edetate
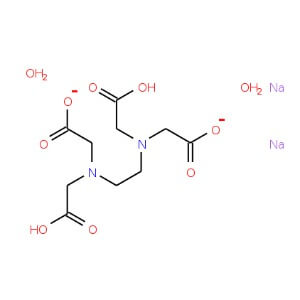
CAS NO: 6381-92-6
MOLECULAR FORMULA :- C10H14N2Na2O82H2O
MOLECULAR WEIGHT :- 372.24 g/ mol
SPECIFICATION IP BP USP Description A white ,crystalline powder, Odourless White or almost white, crystalline powder. White or almost white, crystalline powder. Solubility – Soluble in water, practically insoluble in ethanol (96 %) – Identification A. IR
B. Reaction
C. Reaction
D. Reaction
A. Must comply with the disodium edetate RS
B. No precipitate is produced.
C. No precipitate is produced.
D. It gives reaction of sodium salt.
A. Must comply with the disodium edetate RS
B. No precipitate is produced.
C. No precipitate is produced.
D. It gives reaction of sodium salt.
A. Must comply with the disodium edetate RS
B. The red color is discharge, leaving a yellowish solution
C. It gives reaction of sodium salt.
Appearance of solution 5 % w/v solution in carbon dioxide free water is Clear & colorless Clear & colorless – pH 4.0-5.5 ( 5 % w/v solution ) 4.0-5.5 ( 5 % w/v solution ) 4.0-6.0 Loss on Drying – – 8.7 %-11.4 % Calcium – – No precipitate is formed. Impurity A Limit of nitrilotriacetic acid(USP)
NMT 0.1 % NMT 0.1 % NMT 0.1 % Heavy metals NMT 20 ppm – NMT 50 ppm Iron NMT 80 ppm NMT 80 ppm – Assay 98.5 %-101.0 % 98.5 %-101.0 % 99.0 %-101.0 %(on Dried basis) Ferric Ammonium Citrate
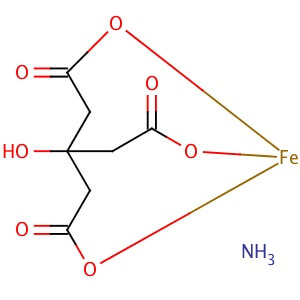
CAS NO: 1185-57-5
MOLECULAR FORMULA : C6H8O7•xFe(III)•yNH3
MOLECULAR WEIGHT :
SPECIALITY FINE CHEMICALS (AR / LR / ACR /GR / IP / BP / USP / FSSAI )Ferrous Sulfate Heptahydrate
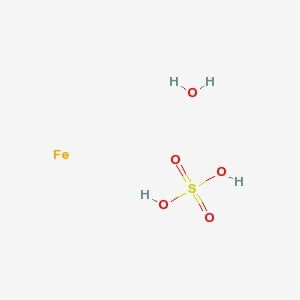
CAS NO: 7782-63-0
MOLECULAR FORMULA :- FeSO4.7H2O
MOLECULAR WEIGHT :- 278.02 g/mol
SPECIFICATION IP BP USP Description Bluish green crystals or a light green, crystalline powder, odourless.Efforescent in air. On exposure To moist air, the crystals rapidly oxidize and become brown. Bluish green crystals or a light green, crystalline powder,efforescent in air. Bluish green crystals or a light green, crystalline powder, odourless.Efforescent in air. Solubility – Freely soluble in water, very soluble in boiling water, practically insoluble in ethanol (96 %) Ferrous sulfate heptahydrate is oxidized in moist air, becoming brown. – pH 3.0-4.0 ( 5 % w/v Solution) 3.0-4.0 – Appearance of Solution The solution is not more opalescent than opalescent Standard. – – Identification (A) Reaction
(B) Reaction
(C) Assay
A. It gives reaction of sulfates B. It gives reaction of Ferrous Salt
A. It gives reaction of sulfates B. It gives reaction of Irons
C. It complies with limit of assay.
A. It gives reaction of sulfates B. It gives reaction of Ferrous Salt
Chloride NMT 250 ppm NMT 200 ppm – Basic sulphate Producing solution that is not more than turbid. – – Arsenic NMT 2 ppm – NMT 3 ppm Lead NMT 50 ppm – NMT 10 ppm Mercury – – It meets the requirements of the test for mercury Chromium – NMT 50 ppm – Copper NMT 50 ppm NMT 50 ppm – Ferric ions NMT 0.5 % NMT 0.3 % – Manganese NMT 0.1 % NMT 0.1 % – Nickel – NMT 50 ppm – Zinc NMT 500 ppm NMT 50 ppm – Organic volatile impurities – – Meets the requirements. Residual Solvents – – Meets the requirements. Assay 98.0 % – 105.0 % 98.0 % – 105.0 % 99.5% -104.5 % Magnesium Chloride Hexahydrate
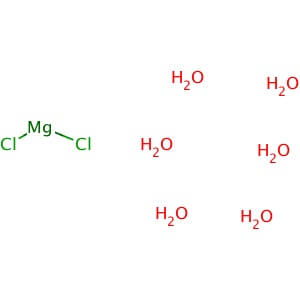
CAS NO: 7791-18-6
MOLECULAR FORMULA: MgCl2•6H2O
MOLECULAR WEIGHT: 203.30
Application: Magnesium Chloride, Hexahydrate is a source of magnesium ion and a co-foactor for many enzymes Purity: >99% Molecular Weight: 203.3 Molecular Formula: MgCl2•6H2O Description: Magnesium Chloride, Hexahydrate is widely used as a source of magnesium ion in chemistry and molecular biology applications. In biological systems, magnesium is a co-factor for many enzymes including deoxyribonuclease (DNase) and various restriction enzymes. Also plays a role in cell membrane integrity, muscle cell physiology, cardiovascular and muscular activity, and nucleic acid structure. Magnesium chloride solution is a favorable choice as an elution buffer for antibody affinity column purifications; it is much milder on most antigens than acid elution, allowing reuse of the antigen column. Also an essential cofactor for the DNA polymerase in polymerase chain reaction (PCR) amplification. Physical State : Solid Solubility : Soluble in water (20 mg/ml), and alcohol. Storage : Store at room temperature Melting Point : 116-118° C (dec.) Density : 1.57 g/cm3 at 20° C Magnesium Sulfate Heptahydrate
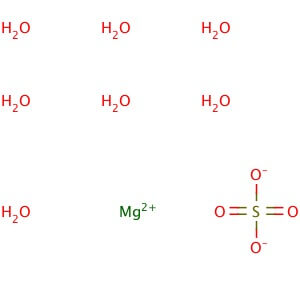
CAS NO: 10034-99-8
MOLECULAR FORMULA :- MgSO4.7H20
MOLECULAR WEIGHT :- 246.50 g/mol
SPECIFICATION IP BP USP Description Colorless crystals or white, Crystalline powder. White or almost white, crystalline powder or brilliant , colorless crystals. Colorless crystals or white, Crystalline powder Solubility – Freely Soluble in water , very soluble in boiling water and practically insoluble in ethanol (96 % )
Identification (A) Reaction
(B) Reaction
A : Reactions of Sulphates B : Reactions of Magnesium salts
A: It gives reaction of Sulphates. B :It gives reaction of magnesium
A: It gives reaction of Sulphates. B : It gives reaction of magnesium
pH of a 5% solution at 250C – – 5.0 – 9.2 Appearance of solution Dissolve 5 g in sufficient carbon dioxide free water to produced 50 ml (Solution A) is clear and colorless. Dissolve 5 g in sufficient carbon dioxide free water to produced 50 ml (Solution S) is clear and colorless. – Acidity or alkalinity NMT 0.2 ml of either 0.01 M hydrochloric acid or 0.01 M sodium hydroxide is required to change the color of solution. NMT.0.2 ml of 0.01 M hydrochloric or 0.01 M sodium hydroxide is required to change the color of indicator. – Arsenic NMT 2 ppm.
NMT 2 ppm – Heavy metals NMT 10 ppm.
– NMT 10 ppm Selenium – NMT 30 ppm Iron NMT 200 ppm. NMT 20 ppm NMT 20 ppm Chloride NMT. 300 ppm NMT. 300 ppm
NMT. 140 ppm Organic Volatile impurities – Meets the requirement Residual Solvents – Meets the requirement Loss on drying 48.0 to 52.0 %
48.0 % – 52.0 % NMT 2.0 % Loss on Ignition – – 40.0%-52.0% Assay (on dried basis) 99.0 % to 100.5 % 99.0 % – 100.5 % 99.0%- 100.5% Potassium Acetate
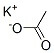
CAS NO: 127-08-2
MOLECULAR FORMULA :- C2H3KO2
MOLECULAR WEIGHT :- 98.14 g/mol
SPECIFICATION BP USP Description White or almost white, crystalline powder or colorless cryastals,deliquescent. White or almost white, crystalline powder or colorless cryastals,deliquescent. Solubility Very soluble in water, freely soluble in ethanol (96 %) – Identification A. Reaction
B. Reaction
A. Reaction of acetates.
B. Reaction of potassium
A. Reaction of acetates.
B. Reaction of potassium
Appearance of solution Clear & Colorless – pH 7.5-9.0 7.5-8.5 Heavy metals – NMT 20 ppm Limit of Sodium – NMT 0.03 % Reducing substances The solution remains pink. – Chloride NMT 200 ppm – Sulfates NMT 200 ppm – Aluminum NMT 1 ppm – Iron NMT 20 ppm – sodium NMT 0.5 % – Loss on drying NMT 3.0 % NMT 1.0 % Residual Solvents – Meets the requirements Assay 99.0 %-101.0 % 99.0%-100.5 % Potassium Carbonate Anhydrous
CAS NO: 584-08-7
MOLECULAR FORMULA :- K2CO3
MOLECULAR WEIGHT :- 138.21 g/mol
SPECIFICATION BP USP Description White or almost white granular Powder hygroscopic. White or almost white granular Powder hygroscopic. Solubility Freely Soluble in water, practically insoluble in Ethanol (96%) – Identification (A) Reaction
(B) Reaction
(C) Reaction
A : The solution is strongly alkaline B: Test A gives the reaction of Carbonates &Bicarbonates.
C: Test A gives reaction of Potassium.
A: Test A gives the reaction of Carbonates. B: Test A gives reaction of Potassium.
Insoluble substances – The solution is complete, clear & Colorless. Heavy metals – NMT 5 ppm Calcium NMT 100 ppm – Iron NMT 10 ppm – Chlorides NMT 100 ppm – Loss on Drying NMT 5.0 % NMT 0.5 % Sulphates NMT 100 ppm – Organic Volatile Impurities – Meets the requirement Residual Solvents – Meets the requirement Assay 99.0 % – 101 % (on dry basis) 99.5 % – 100.5 % Potassium Chloride
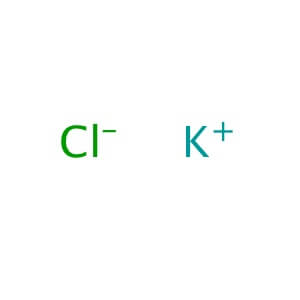
CAS NO: 7447-40-7
MOLECULAR FORMULA: KCl
MOLECULAR WEIGHT: 74.55
Alternate Names: Potassium Chloride is also known as KCl. Application: Potassium Chloride is a common laboratory reagent and calibration standard for measuring electrical conducivity. Purity: >99% Description: Potassium Chloride is commonly used as a laboratory reagent as a standard. Potassium Chloride may be used as a calibration standard ionic solution for measuring electrical conductivity because Potassium Chloride solutions, when prepared carefully, produce repeatable measurable properties. Potassium Chloride is also a good source of ionic chloride, which should precipitate insoluble chloride salts upon addition to a solution of an appropriate metal ion. Oral Potassium Chloride is commonly used to replenish vital potassium in the body, and has also been shown to lower blood pressure. Potassium Chloride is commonly used in fertilizer. Physical State : Solid Solubility : Soluble in water (340 mg/ml at 20° C), alcohol (slightly), and ethanol (1 g/250 mL). Insoluble in ether, and acetone. Storage : Store at room temperature Melting Point : 770° C (lit.) Boiling Point : 1500° C (lit.) Density : 1.98 g/cm3 at 25° C (lit.) Refractive Index : n20D 1.49 Ki Data : CA IV: Ki= 90 µM (human); CA I: Ki= 6 mM (human); CA IX: Ki= 33 mM (human); CA V: Ki= 156 mM (human); CA II: Ki>200 mM (human)
Showing 1–24 of 56 items