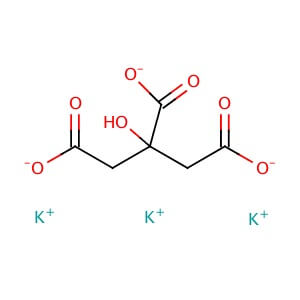
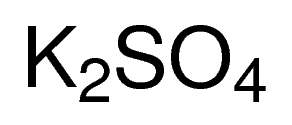Potassium Citrate
CAS NO: 866-84-2
MOLECULAR FORMULA: C6H5K3O7
MOLECULAR WEIGHT: 306.39
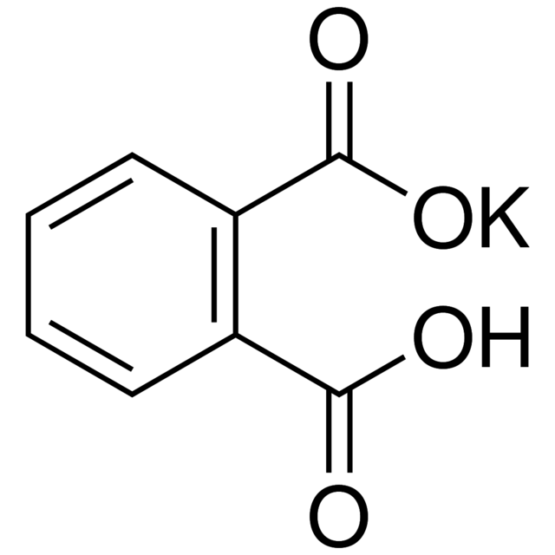
SPECIALITY FINE CHEMICALS (AR / LR / ACR /GR / IP / BP / USP / FSSAI )Potassium Hydrogen Phthalate
CAS NO: 877-24-7
MOLECULAR FORMULA : C8H5KO4
MOLECULAR WEIGHT : 204.22
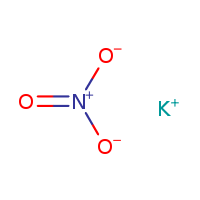
SPECIALITY FINE CHEMICALS (AR / LR / ACR /GR / IP / BP / USP / FSSAI )Potassium Nitrate
CAS NO: 7757-79-1
MOLECULAR FORMULA :- KNO3
MOLECULAR WEIGHT :- 101.1g/mol
SPECIFICATION BP USP Description White or almost white, crystalline powder or colorless crystals. White or almost white, crystalline powder or colorless crystals. Solubility Freely soluble in water, very soluble in boiling water, practically insoluble in ethanol (96 %) – Identification A. Reaction
B. Reaction
A. It gives reaction of nitrates. B. It gives reaction of Potassium
A. It gives reaction of nitrates. B. It gives reaction of Potassium
Acidity or Alkalinity NMT 0.5 ml of 0.01 M HCl or 0.01 M NaOH is required to change the color of the indicator. – Reducible Substances The solution does not become blue within 2 min. – Chlorides NMT 20 ppm NMT 300 ppm Sulfates NMT 150 ppm NMT 1000 ppm Limit of Nitrite – NMT 5 ppm Heavy metals – NMT 20 ppm Arsenic – NMT 3 ppm Lead – NMT 10 ppm Ammonium NMT 100 ppm – Calcium NMT 100 ppm – Iron NMT 20 ppm NMT 10 ppm Residual Solvents – Meets the requirements. Sodium NMT 1000 ppm NMT 1000 ppm Loss on Drying NMT 0.5 % – Assay 99.0 %-101.1 % (on dried basis) 99.0 %-100.5 % Potassium Phosphate Monobasic
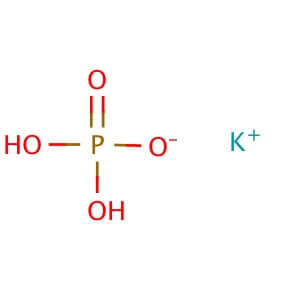
CAS NO: 7778-77-0
MOLECULAR FORMULA :- KH2PO4
MOLECULAR WEIGHT :- 136.10 g/mol
SPECIFICATION IP BP USP Description White or almost white powder , crystalline powder , colorless powder or crystalline masses, efflorescent. White or almost white powder , crystalline powder , colorless powder or crystalline masses, efflorescent. Appearance of solution 4% w/v Solution is clear and colorless – Solubility Soluble in water , very soluble in boiling water and freely soluble in glycerol. – Identification (A) Reaction
(B) Reaction
(C) Reaction
A. Solution S is faintly acid
B. Reaction of Potassium
C. Reaction for Phosphates
A. Reaction of Potassium B. Reaction for Phosphates
pH (4% w/v solution in water) 4.2-4.5 – Sulphate NMT 50 ppm.
– Ammonium NMT10 ppm.
– Arsenic NMT 2 ppm. NMT 3 ppm Sodium NMT 1000 ppm – Iron NMT 10 ppm – Sulphate NMT 300 ppm – Chloride NMT 200 ppm – Lead – NMT 5 ppm Heavy Metal – NMT 20 ppm Fluoride – NMT 10 ppm Reducing Substances The colour of permanganate is not completely discharged – Residual Solvents – Meets the requirement Organic volatile impurities – Meets the requirement Insoluble substances – Max. 0.2% Loss on Drying at 1050C Max. 2.0% Max. 1.0% Assay Na2B4O7,10H2O 98.0 % – 100.5 98.0 % to 100.5 % Potassium Sulphate
CAS NO: 7778-80-5
MOLECULAR FORMULA : K2SO4
MOLECULAR WEIGHT : 174.26
SPECIALITY FINE CHEMICALS (AR / LR / ACR /GR / IP / BP / USP / FSSAI )Potassium Sulphate Crystals
CAS NO: 7778-80-5
MOLECULAR FORMULA : K2SO4
MOLECULAR WEIGHT : 174.26
SPECIALITY FINE CHEMICALS (AR / LR / ACR /GR / IP / BP / USP / FSSAI )
Showing 25–30 of 56 items