Description
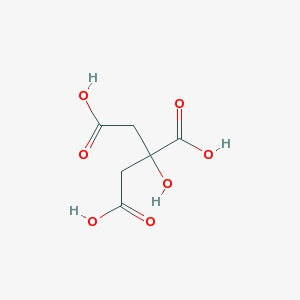
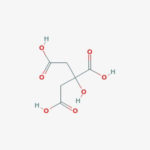
Citric Acid is an acidic compound from citrus fruits; as a starting point in the Krebs cycle, citrate is a key intermediate in metabolism. Citric acid is one of a series of compounds responsible for the physiological oxidation of fats, carbohydrates and proteins to carbon dioxide and water. Often used to prepare citrate buffer for antigen retrieval of tissue samples. The citrate solution is designed to break protein cross-links; thus, unmasking antigens and epitopes in formalin-fixed and paraffin embedded tissue sections, resulting in enhancing staining intensity of antibodies. Citrate has anticoagulant activity; as a calcium chelator, it forms complexes that disrupt the tendency of blood to clot. May be used to adjust pH and as a sequestering agent for the removal of trace metals.


Reviews
There are no reviews yet.